7th & 8th MAY, 2025
San Francisco, CA
Advancing hit discovery for complex and emerging targets is revolutionizing the landscape of modern drug development, transforming the pursuit of novel therapies. As we delve into the realm of intricate biological targets, such as protein-protein interactions, allosteric sites, and epigenetic modulators, the challenges are formidable, but the potential rewards are immense. These targets, once considered elusive, are now within reach, thanks to innovative approaches and cutting-edge technologies.
The complexity of these targets demands a paradigm shift in hit discovery strategies. Traditional high-throughput screening (HTS) methods, while still foundational, are being augmented by advanced techniques like structure-based drug design (SBDD), fragment-based drug discovery (FBDD), and the transformative power of artificial intelligence (AI). SBDD harnesses the intricacies of target structures to craft more precise and potent compounds, while FBDD deconstructs potential drugs into smaller, more manageable fragments for systematic optimization. AI and machine learning algorithms further accelerate this process by predicting molecular interactions with unprecedented accuracy and speed.
Crucially, the adoption of physiologically relevant assays is enhancing the fidelity of early-stage drug discovery. Traditional in vitro assays often fall short in replicating the complexity of living systems, leading to high attrition rates. Innovative approaches, such as cell-based assays and organ-on-a-chip models, provide a more accurate representation of how compounds will perform in vivo, thereby improving the quality and relevance of identified hits.
Collaboration stands as a cornerstone of this advancement. Partnerships across academia, industry, and technology sectors bring together diverse expertise and state-of-the-art resources, propelling the discovery process forward. Shared data, insights, and methodologies amplify our collective ability to address complex targets and drive breakthroughs.
In conclusion, the advancement of hit discovery for complex and emerging targets is a multifaceted endeavor that marries advanced technologies, realistic assays, and collaborative synergy. By embracing these strategies, we are poised to unlock new therapeutic frontiers, delivering groundbreaking treatments for challenging diseases and transforming the future of medicine.
Incorporating the patient voice into the design of clinical trials marks a transformative approach in medical research, fundamentally shifting the focus towards more patient-centric outcomes. Historically, clinical trials have been primarily shaped by researchers and clinicians, often overlooking the critical insights that patients bring to the table. Including patient perspectives is essential because it ensures that trials address the real-world concerns and outcomes that matter most to those living with the condition. Patients can provide unique insights into their daily challenges, treatment preferences, and quality-of-life considerations, leading to more relevant and effective study designs that enhance the likelihood of successful outcomes and patient adherence.
Engaging patients early in the trial design process is crucial. By involving them during the initial stages, such as protocol development, selection of outcome measures, and recruitment strategies, researchers can identify potential barriers and enhance the study’s feasibility and relevance. Continuous engagement throughout the trial can also provide valuable feedback and ensure the study remains patient-centered. There are several effective strategies for incorporating patient input, including forming patient advisory boards to provide ongoing feedback and guidance, conducting surveys and focus groups to gather diverse patient perspectives, collaborating with patient advocacy groups to leverage their networks and expertise, and including patients as members of trial steering committees to ensure their views are represented in decision-making processes. Ultimately, integrating the patient voice in clinical trial design enhances the relevance, feasibility, and success of medical research. By listening to and valuing patient input, researchers can create more effective and patient-centered studies, leading to better health outcomes and advancing medical research in a meaningful way.
Flipping the dialogue on the clinical trial industry’s true expectations from service providers reveals a fresh perspective on what is genuinely needed for success. Traditionally, the focus has been on what service providers, like Contract Research Organizations (CROs), expect from clinical trial sponsors. However, it’s crucial to understand what sponsors truly need from their service providers to ensure the smooth execution and ultimate success of clinical trials.
At the forefront, sponsors value transparency and open communication. Clear, consistent updates on trial progress, challenges, and changes are vital. Sponsors need to feel confident that they are fully informed and that any issues are promptly addressed. This level of transparency builds trust and ensures that all stakeholders are on the same page.
Expertise and experience in specific therapeutic areas are also highly valued. Sponsors look for service providers who have a proven track record in handling similar trials. This specialized knowledge can significantly enhance the trial’s efficiency and effectiveness, reducing the risk of unforeseen issues and ensuring high-quality outcomes.
Flexibility and adaptability are equally important. Clinical trials are complex and often unpredictable. Sponsors need service providers who can pivot and adjust plans as necessary without compromising the trial’s integrity. This flexibility helps navigate the inevitable challenges and keeps the trial on track.
Moreover, sponsors expect innovation from their service providers. Utilizing the latest technologies and methodologies can streamline processes, reduce costs, and improve data quality. Innovative approaches demonstrate a provider’s commitment to excellence and forward-thinking.
Finally, a collaborative approach is essential. Sponsors want to feel like partners rather than clients. A collaborative relationship fosters a sense of shared responsibility and mutual goals, leading to more productive and successful outcomes.
In summary, flipping the dialogue to understand what clinical trial sponsors expect from service providers highlights the importance of transparency, expertise, flexibility, innovation, and collaboration. By meeting these expectations, service providers can significantly enhance the success and efficiency of clinical trials.
Revolutionizing sustainable cold chain packaging for high-volume shipments is crucial in today’s world, where environmental impact and efficiency are top priorities. The cold chain, essential for transporting temperature-sensitive goods like pharmaceuticals and perishable foods, has traditionally relied on packaging materials that are often wasteful and harmful to the environment. However, innovative solutions are emerging to address these challenges, making the cold chain more sustainable and cost-effective.
One of the key advancements in this field is the development of eco-friendly packaging materials. Companies are now using biodegradable and recyclable materials that offer the same level of thermal protection as traditional options. These materials not only reduce the environmental footprint but also help companies meet stringent regulatory standards and consumer expectations for sustainability.
Another significant innovation is the use of advanced insulation technology. New insulation materials and designs provide superior temperature control, ensuring that products remain within the required temperature range throughout transit. This not only preserves the quality and safety of the goods but also reduces the amount of energy needed to maintain cold temperatures, further cutting down on carbon emissions.
Reusable packaging solutions are also gaining traction. Instead of single-use containers, companies are investing in durable, reusable options that can withstand multiple shipping cycles. This not only reduces waste but also lowers overall packaging costs in the long run.
Digital technologies are playing a vital role as well. IoT sensors and smart tracking systems provide real-time monitoring of temperature conditions, ensuring immediate corrective actions if any deviations occur. This not only enhances the reliability of the cold chain but also minimizes product loss and waste.
In conclusion, the revolution in sustainable cold chain packaging for high-volume shipments is driven by eco-friendly materials, advanced insulation, reusable solutions, and smart technology. These innovations are paving the way for a more sustainable, efficient, and reliable cold chain, benefiting businesses, consumers, and the planet.
Abstract
High failure rates in clinical trials remain a challenge in drug development. Many investigational compounds fail in the phases of clinical trials. Especially in Phase II due to a lack of target reaching and binding, low specificity, or side effects. Integrating imaging in in-patient clinical trials offers a promising solution. Valuable data on pharmacokinetics and biodistribution for informed decision-making in Phase 2, can already be obtained in Phase 0 exploratory trials. Regulatory bodies support this approach, encouraging its adoption to expedite drug development and improve patient outcomes.
Obtaining market approval for your drug, that’s the destination. Why follow a path that will almost certainly lead to failure? It is common knowledge in drug development that most investigational new drugs fail in clinical trials. To be more specific, the failure rate of new drugs has been around 90% in clinical trials for years. Despite the work of experienced professionals and regulatory authorities, the industry is still looking for solutions to overcome this challenge. In this article, we zoom in on a trend that gains ground and could potentially lead to change: imaging in clinical trials, and especially nuclear (PET or SPECT) imaging in a Phase 0 clinical trial.
The impact of imaging on clinical trials is versatile. Before we go into depth, let’s look at the outlines.
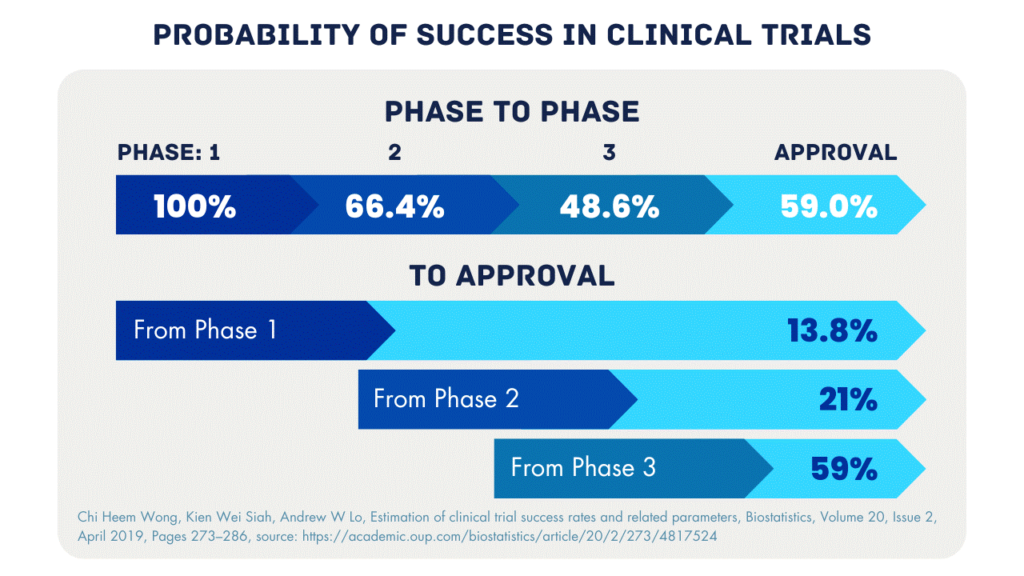
Percentages indicate the probability of success in moving to the next phase and the success rate from each stage to approval.
As can be seen in the figure above, most compounds fail in Phase 2. A lack of specificity is one of the main reasons for failure. The compound does not reach or bind to the target or there is too much off-target binding. Phase 0 is a great opportunity for drug developers to examine this problem. Even before Phase 1 a microdose of their drug can be administered to patients to study pharmacokinetics and biodistribution. The data from this study can be used to optimize the compound, select a lead compound, and design the Phase 2 study to increase the chance of success.
The use of imaging, such as CT or MRI to measure tumor size, is already well-established in clinical trials. Nuclear imaging (e.g., PET and SPECT) and optical imaging (e.g., fluorescence) are gaining ground. They can be employed in clinical trials for molecular and functional analysis. Pharmacokinetics and biodistribution of a new drug can be visualized with imaging. Depending on the imaging agent and the compound, multiple imaging timepoints can be implemented to monitor the on- and off-target uptake and excretion over time.
Imaging is a non-invasive procedure. However, imaging can be cross-correlated for target expression via a fresh or previously taken and stored biopsy. PET and SPECT are ideal at a macro level. Fluorescent imaging is more suited to provide data on a cellular level. The limited penetration depth of several centimeters is worth mentioning for fluorescent imaging. Multiple imaging methods can be combined in a dual-labeling strategy to gain a comprehensive understanding of patient profiles, biomarkers, and the mechanism of action. Since in a Phase 0 study only a microdose of the compound is administered to the patient, the dose is too low to measure pharmacological effects. This makes imaging ideal for Phase 0 because a small dose is already sufficient to visualize the targeting of the compound.
Phase 0 is a rapid, first-in-human clinical trial that is conducted before Phase I. During this early exploratory phase, a microdose/non-therapeutic dose of the new drug is administered to a small group of patients, typically less than 15 subjects. The low dose allows drug developers to bypass extensive preclinical research and full toxicology studies.
It is highly recommended to add imaging to a Phase 0 study, this way the compound can be tracked through the body. This can especially be interesting for intravenous administration. There is also a practical benefit of imaging, the compound may be of GLP grade. Labeling the drug under GMP conditions makes it suitable for clinical use.
A part of failed studies, and therefore, discontinued programs are due to a lack of on-target binding or the result of too much off-target binding. Imaging can visualize target binding. Unraveling the binding mechanism can lead to a higher success rate in clinical trials. The clinical trial design includes indications, inclusion criteria, and endpoints. A comprehensive understanding of a drug helps in making the right decisions.
In Phase 0, the proof of concept is obtained by imaging target reaching and binding. This provides information to determine the relation between indication, target identification, and lead compound selection. Basket trials are well suited for this; you broaden your scope first and then select the most suitable indication expressing your target. Phase 0 in-patient studies can substitute studies with large animal models. Phase 0 data can be considered more valuable than large animal data due to variations in PK/BD across different species.
Together with TRACER, an imaging CRO specialized in Phase 0, we answer some practical questions on this topic.
This depends on when Phase 0 is initiated; if it replaces part of the preclinical work, it can save time. Moreover, data obtained from Phase 0 can be used to accelerate subsequent trials. If a program stagnates due to a lack of funding, Phase 0 can accelerate that process as well. The funding needed for Phase 0 is generally lower, and Phase 0 is risk-mitigating. The needed resources for Phase 0 can often be reallocated from late-preclinical to early-clinical. For example, as large animal models can be omitted, this budget can be used to fund the Phase 0 study.
Yes, Phase 0, and microdosing, is approved by the FDA and EMA. Both regulatory bodies encourage the industry to use innovations in clinical trials to combat the high failure rate and speed up the process of getting new drugs to patients.
Ranging from antibodies to peptides to small molecules, most compounds are suitable for labeling. The imaging agent and imaging technique should be chosen depending on the compound and indication. You can discuss the applicable options for your drug development directly with TRACER. Discuss possibilities for your clinical trial with TRACER CRO.
The cost of a Phase 0 trial depends on the study design. By submitting your request for proposal at TRACER, you will receive an estimate of the budget, study contents, and timelines.
Phase 0 and imaging in drug development show promise for higher success rates. However, this is only true if the industry deploys this strategy. For drug developers, this means considering Phase 0 as the first-in-human trial and adding imaging endpoints to other clinical trials. For investors, it could mean making Phase 0 a prerequisite for funding as a risk-mitigating measure.
When it comes to medical research, involving the community is crucial. Imagine a puzzle – each piece represents a different part of the community coming together to support research. This collaboration is essential for the success of clinical trials, where new medicines and treatments are tested.
Community engagement means involving people from different backgrounds and experiences in the research process. It’s like inviting friends over to help solve the puzzle. Their unique perspectives and insights can make the research stronger and more effective.
One important aspect of community engagement is supporting investigative sites. These are the places where clinical trials take place, like hospitals or clinics. By involving the local community, these sites can better understand the needs and concerns of the people participating in the trials. This makes the research more ethical and respectful.
Another benefit of community engagement is promoting trial diversity. Just like a puzzle is more interesting with different colors and shapes, clinical trials are more effective when they include a diverse group of people. This ensures that the medicines and treatments being tested work for everyone, regardless of their background or circumstances.
When the community is involved in clinical trials, everyone benefits. Participants feel more supported and understood, which encourages them to stay involved. Researchers get valuable insights and feedback, which helps them improve their work. And ultimately, new medicines and treatments can be developed that benefit the whole community.
In conclusion, community engagement is like the missing piece of the puzzle in clinical research. By involving people from different backgrounds and experiences, we can build stronger, more effective trials that benefit everyone. So let’s come together and support medical research in our communities!
In the realm of temperature-controlled logistics, where maintaining precise conditions is paramount, innovation and creativity play pivotal roles in driving efficiency and effectiveness. As industries evolve and consumer demands shift, it becomes imperative to evaluate and adopt methods that not only meet regulatory requirements but also exceed expectations in terms of reliability and sustainability.
One innovative approach lies in the integration of advanced monitoring and analytics systems. Real-time monitoring using IoT devices combined with sophisticated analytics allows for proactive identification of potential issues, enabling swift interventions to prevent temperature excursions or product spoilage. This not only reduces losses but also enhances overall supply chain efficiency.
Furthermore, the utilization of blockchain technology can revolutionize temperature-controlled logistics by ensuring transparency and traceability throughout the supply chain. With blockchain, stakeholders can securely access immutable records of temperature data, ensuring compliance with stringent regulations and providing consumers with confidence in product integrity.
Additionally, leveraging automation and robotics in temperature-controlled warehouses can significantly improve operational efficiency. Automated storage and retrieval systems, coupled with robotic palletizing and picking, streamline processes, minimize errors, and expedite order fulfillment, ultimately reducing lead times and enhancing customer satisfaction.
Moreover, embracing renewable energy sources and eco-friendly refrigerants aligns with sustainability goals while reducing carbon footprint. From solar-powered refrigeration units to the adoption of natural refrigerants like CO2 or ammonia, innovative solutions contribute to environmental conservation without compromising temperature control standards.
In conclusion, the pursuit of innovation and creativity in temperature-controlled logistics is essential for enhancing efficiency and effectiveness. By embracing technological advancements, ensuring transparency, automating processes, and prioritizing sustainability, businesses can optimize their operations and deliver superior outcomes in an ever-evolving landscape of logistical challenges.