Abstract
High failure rates in clinical trials remain a challenge in drug development. Many investigational compounds fail in the phases of clinical trials. Especially in Phase II due to a lack of target reaching and binding, low specificity, or side effects. Integrating imaging in in-patient clinical trials offers a promising solution. Valuable data on pharmacokinetics and biodistribution for informed decision-making in Phase 2, can already be obtained in Phase 0 exploratory trials. Regulatory bodies support this approach, encouraging its adoption to expedite drug development and improve patient outcomes.
Imaging: the missing piece in clinical trials
Obtaining market approval for your drug, that’s the destination. Why follow a path that will almost certainly lead to failure? It is common knowledge in drug development that most investigational new drugs fail in clinical trials. To be more specific, the failure rate of new drugs has been around 90% in clinical trials for years. Despite the work of experienced professionals and regulatory authorities, the industry is still looking for solutions to overcome this challenge. In this article, we zoom in on a trend that gains ground and could potentially lead to change: imaging in clinical trials, and especially nuclear (PET or SPECT) imaging in a Phase 0 clinical trial.
How can imaging improve clinical trial success rates?
The impact of imaging on clinical trials is versatile. Before we go into depth, let’s look at the outlines.
- Firstly, by adding imaging, new clinical endpoints become available.
- Secondly, by combining imaging with Phase 0 – the exploratory phase in drug development – an early go-/no-go decision on further development of the investigated compound can be made.
- Thirdly, imaging sheds light on on- and off-target binding.
Most compounds fail in Phase 2
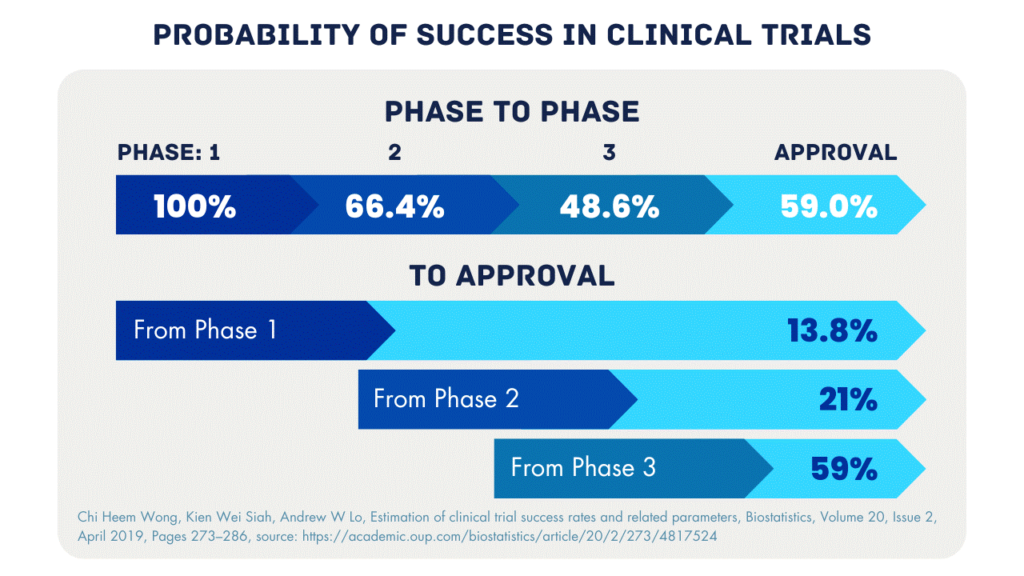
Percentages indicate the probability of success in moving to the next phase and the success rate from each stage to approval.
As can be seen in the figure above, most compounds fail in Phase 2. A lack of specificity is one of the main reasons for failure. The compound does not reach or bind to the target or there is too much off-target binding. Phase 0 is a great opportunity for drug developers to examine this problem. Even before Phase 1 a microdose of their drug can be administered to patients to study pharmacokinetics and biodistribution. The data from this study can be used to optimize the compound, select a lead compound, and design the Phase 2 study to increase the chance of success.
Clinical endpoints defined by imaging
The use of imaging, such as CT or MRI to measure tumor size, is already well-established in clinical trials. Nuclear imaging (e.g., PET and SPECT) and optical imaging (e.g., fluorescence) are gaining ground. They can be employed in clinical trials for molecular and functional analysis. Pharmacokinetics and biodistribution of a new drug can be visualized with imaging. Depending on the imaging agent and the compound, multiple imaging timepoints can be implemented to monitor the on- and off-target uptake and excretion over time.
Combining multiple imaging methods in one clinical trial
Imaging is a non-invasive procedure. However, imaging can be cross-correlated for target expression via a fresh or previously taken and stored biopsy. PET and SPECT are ideal at a macro level. Fluorescent imaging is more suited to provide data on a cellular level. The limited penetration depth of several centimeters is worth mentioning for fluorescent imaging. Multiple imaging methods can be combined in a dual-labeling strategy to gain a comprehensive understanding of patient profiles, biomarkers, and the mechanism of action. Since in a Phase 0 study only a microdose of the compound is administered to the patient, the dose is too low to measure pharmacological effects. This makes imaging ideal for Phase 0 because a small dose is already sufficient to visualize the targeting of the compound.
Phase 0
Phase 0 is a rapid, first-in-human clinical trial that is conducted before Phase I. During this early exploratory phase, a microdose/non-therapeutic dose of the new drug is administered to a small group of patients, typically less than 15 subjects. The low dose allows drug developers to bypass extensive preclinical research and full toxicology studies.
Advantages of imaging
It is highly recommended to add imaging to a Phase 0 study, this way the compound can be tracked through the body. This can especially be interesting for intravenous administration. There is also a practical benefit of imaging, the compound may be of GLP grade. Labeling the drug under GMP conditions makes it suitable for clinical use.
Keypoints on Phase 0:
- It is a relatively quick clinical trial.
- It may take only between 12-18 months from study start to study closure.
- Regulatory approval is quick.
- The study is relatively low budget.
- Limited patients are enrolled (n= 10-15).
- Single-dose extended toxicity study and GLP material are sufficient, making large investments in GMP material and a full-tox study at this stage unnecessary.
- The in-patient data obtained from Phase 0 provides information for a smarter design of subsequent clinical trials and allows for rapid identification of the lead candidate and target.
On and off-target binding
A part of failed studies, and therefore, discontinued programs are due to a lack of on-target binding or the result of too much off-target binding. Imaging can visualize target binding. Unraveling the binding mechanism can lead to a higher success rate in clinical trials. The clinical trial design includes indications, inclusion criteria, and endpoints. A comprehensive understanding of a drug helps in making the right decisions.
Obtain data for your study design
In Phase 0, the proof of concept is obtained by imaging target reaching and binding. This provides information to determine the relation between indication, target identification, and lead compound selection. Basket trials are well suited for this; you broaden your scope first and then select the most suitable indication expressing your target. Phase 0 in-patient studies can substitute studies with large animal models. Phase 0 data can be considered more valuable than large animal data due to variations in PK/BD across different species.
Practical questions about Phase 0 and imaging in clinical trials
Together with TRACER, an imaging CRO specialized in Phase 0, we answer some practical questions on this topic.
Does Phase 0 lengthen the drug development process?
This depends on when Phase 0 is initiated; if it replaces part of the preclinical work, it can save time. Moreover, data obtained from Phase 0 can be used to accelerate subsequent trials. If a program stagnates due to a lack of funding, Phase 0 can accelerate that process as well. The funding needed for Phase 0 is generally lower, and Phase 0 is risk-mitigating. The needed resources for Phase 0 can often be reallocated from late-preclinical to early-clinical. For example, as large animal models can be omitted, this budget can be used to fund the Phase 0 study.
Has Phase 0 been approved by the FDA and EMA?
Yes, Phase 0, and microdosing, is approved by the FDA and EMA. Both regulatory bodies encourage the industry to use innovations in clinical trials to combat the high failure rate and speed up the process of getting new drugs to patients.
Can any compound be labeled for imaging?
Ranging from antibodies to peptides to small molecules, most compounds are suitable for labeling. The imaging agent and imaging technique should be chosen depending on the compound and indication. You can discuss the applicable options for your drug development directly with TRACER. Discuss possibilities for your clinical trial with TRACER CRO.
What are the costs of a Phase 0 trial?
The cost of a Phase 0 trial depends on the study design. By submitting your request for proposal at TRACER, you will receive an estimate of the budget, study contents, and timelines.
Conclusion
Phase 0 and imaging in drug development show promise for higher success rates. However, this is only true if the industry deploys this strategy. For drug developers, this means considering Phase 0 as the first-in-human trial and adding imaging endpoints to other clinical trials. For investors, it could mean making Phase 0 a prerequisite for funding as a risk-mitigating measure.